Unlocking speed, accuracy, compliance, and innovation in the clinical trial value chain through Enterprise AI solutions
Clinical trials are the backbone of life sciences and healthcare innovation- but they are also highly complex, slow, expensive, and data-intensive. Life sciences organisations generate an unprecedented amount of data, which usually ranges from Omics data, EHR/EMR systems, lab instruments, medical imaging (DICOM image), genomics platforms, devices and wearables, and patient-reported outcomes- the need for intelligent, governed, and automated data operations has never been more pressing.
This is where Enterprise AI- particularly AI-based data governance, discovery, classification, and readiness solutions- has emerged as a transformative catalyst. Instead of simply applying models to raw or siloed data, Enterprise AI ensures that clinical trial data is accurate, compliant, submission-ready, high-quality, de-duplicated, well-classified, and ready for downstream AI technologies. The shift is profound: organisations are no longer struggling to “prepare data for AI”- they are using AI to prepare data for AI. In other terms, the information architecture should be ready to apply relevant AI-based models and cutting-edge AI technologies.
Why Enterprise AI Matters in Clinical Trials
Traditional clinical trial processes face persistent challenges, vis-à-vis, slow patient recruitment, fragmented data sources, manual documentation, protocol deviations, regulatory burdens, traditional site monitoring mechanisms and unpredictable cycle times. The root cause behind many of these inefficiencies is poor data readiness- data that is incomplete, inconsistent, misclassified, or trapped in legacy silos.
Enterprise AI resolves this by providing:
- Automated data discovery & classification across multi-cloud and on-prem systems
- Metadata enrichment & harmonization for clinical, imaging, and operational datasets
- End-to-end data lineage and governance to improve auditability
- AI-powered retention, quality, and compliance controls
- Secure access, anonymization, and masking for PHI and PII
With foundational data governance powered by AI, organizations can deploy advanced analytics, predictive/ prescriptive modelling, NLP systems, and generative AI with dramatically higher accuracy and operational efficiency.
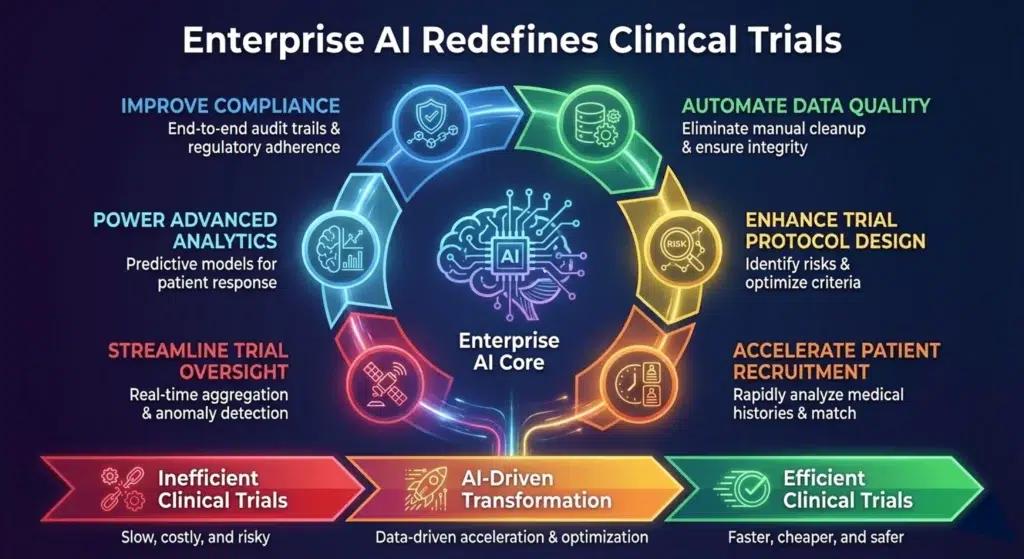
Key Ways Enterprise AI Is Redefining Clinical Trial Operations
1. Accelerating Patient Recruitment, Analysing Site Feasibility & Eligibility Matching
AI-driven classification and entity extraction allow systems to rapidly analyse medical histories, codes, biomarkers, and clinical notes to identify suitable patients. This, in turn, becomes one of the core components of any decentralized clinical trials.
Enterprise AI ensures:
- Clean and standardized patient datasets
- PII/PHI data preservation through masking and tokenization
- Real-time study eligibility scoring
This accelerates recruitment by a significant amount, thereby, reducing bottlenecks in trial timelines.
2. Enhancing Trial Protocol Design and Study Feasibility
Enterprise AI creates harmonized datasets that fuel statistical insights, predictive/ prescriptive modelling, and simulations. With governed and quality-assured data, research teams can:
- Identify protocol risks before trials begin
- Predict dropout rates
- Optimize site selection
- Model operational scenarios with higher accuracy
3. Automating Data Quality, Cleaning & Standardization
Clinical data arrives in dozens of formats, from lab systems to sensors. Enterprise AI automates:
- Schema mapping
- De-duplication
- Validation checks
- Outlier detection
- Metadata standardization into CDISC/SDTM formats
This eliminates weeks or months of manual data cleanup, ensuring near-real-time trial monitoring.
4. Improving Compliance, Audit Readiness & Data Security
With global regulations like GxP (GMP, GCP, GLP, etc.), HIPAA, GDPR, and 21 CFR Part 11 (for electronic records and e-signatures), clinical trials demand strict governance.
AI-based governance platforms provide:
- End-to-end audit trails
- Automated retention & archival policies
- Real-time anomaly detection
- PHI/PII classification & anonymization
- Secure role-based access
- e-Consenting mapped to the 21 CFR Part 11 regulation
Organizations reduce compliance risk while maintaining data integrity throughout the trial lifecycle.
5. Powering Advanced Analytics, Real World Evidence (RWE), and AI Model Performance
Once datasets are governed and AI-ready, organizations can deploy:
- Predictive analytics for patient response
- NLP for unstructured data to understand semantic similarities (clinical notes, imaging reports)
- Digital twins for trial simulation
- AI-powered risk-based monitoring
- GenAI for medical writing, CSR automation, and insights summarization
The foundation of AI in life sciences is clean, governed, and high-quality data—something Enterprise AI excels at.
6. Streamlining Trial Oversight & Site Monitoring
Enterprise AI enables remote monitoring by:
- Aggregating site data
- Flagging anomalies in patient safety
- Identifying deviations from protocols
- Predicting operational risks
This reduces on-site visits and improves the quality of trial oversight.
Future Outlook: AI-Driven, Data-Optimized Clinical Trials
The next era of clinical research will be defined by intelligent data operations. As organizations embrace Enterprise AI, we can expect:
- Self-governing data ecosystems with automated lineage and stewardship
- Real-time patient insights from wearables and digital health devices
- AI-native clinical trial designs powered by predictive models
- Fully digital, decentralized trial operations
- Faster regulatory submissions with automated document generation
Ultimately, Enterprise AI will reduce timelines, improve accuracy, and make clinical trials more accessible, transparent, and patient-centric.
Conclusion
Enterprise AI is not just improving clinical trial operations—it is rebuilding the ecosystem from the ground up. By ensuring data is governed, trusted, compliant, and AI-ready, Enterprise AI empowers life sciences organizations to accelerate research, reduce costs, and bring life-changing therapies to patients faster.
In a world defined by data complexity, AI in life sciences is the competitive advantage. And the organizations that invest in AI-powered data governance today will lead the clinical research breakthroughs of tomorrow.
Solix enables enterprises to unify, govern, secure, and activate their data so it’s audit-ready, secure, private, and available right out of the box. Our solutions combine compliant archiving, classification, cataloging, data governance policy enforcement, document and file management, and data unification, transforming fragmented content into governed, reusable assets that support analytics and AI.
Solix EAI takes this foundation further. It’s a model-agnostic platform designed to stage governed data and create a production-level RAG system that operates across your Gen-AI environments.With hybrid retrieval, re-ranking, smart chunking, policy-aware access controls (RBAC/ABAC), masking of patient identifiers, protocol-driven legal holds, and audit-grade lineage, Solix EAI enables “stage once, deploy RAG everywhere” across clinical trial study design, site monitoring, Risk-based Quality Management (RBQM), Medical Monitoring, TMF management, pharmacovigilance reviews, and regulatory submissions- delivering precise, citation-backed answers while maintaining strict GxP and data-privacy compliance.